Oncologists May Prescribe New Neutropenia Agent Over Others in Class, but Therapy Faces Another Challenge
Posted on November 16, 2022
Republished with Permission from AIS Health
The FDA recently approved the first novel long-acting granulocyte colony-stimulating factor (G-CSF) in more than 20 years. Payers say they are likely to manage the new agent similar to existing ones, but some oncologists have indicated that they are willing to prescribe it in place of other neutropenia agents, according to Zitter Insights. Still, the leader in the space has a unique quality that has allowed it to continue to retain market share, which may prove challenging for the new drug — at least for the time being.
On Sept. 9, the FDA approved Spectrum Pharmaceuticals, Inc.’s Rolvedon (eflapegrastim-xnst) to decrease the incidence of infection, as manifested by febrile neutropenia, in adults with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile neutropenia. The company developed the drug with South Korea’s Hanmi Pharmaceutical Co. The recommended dose is 13.2 mg administered subcutaneously once per chemotherapy cycle.
On Oct. 21, Spectrum reported that Rolvedon was commercially available. “We are thrilled to launch ROLVEDON into an estimated $2 billion market,” said Tom Riga, president and CEO of Spectrum, in a statement. “Our distribution partners have stocked the product and we are ready to take advantage of this compelling market opportunity.”
Other neutropenia treatments include Amgen Inc.’s Neupogen (filgrastim), first approved in 1991, and Neulasta (pegfilgrastim), its longer-acting formulation approved in 2002 that is the current No. 1 medication in the space, as well as biosimilars of both agents. As of early November 2022, the FDA had approved three Neupogen biosimilars and six of Neulasta, although not all of them had launched as of AIS Health press time.
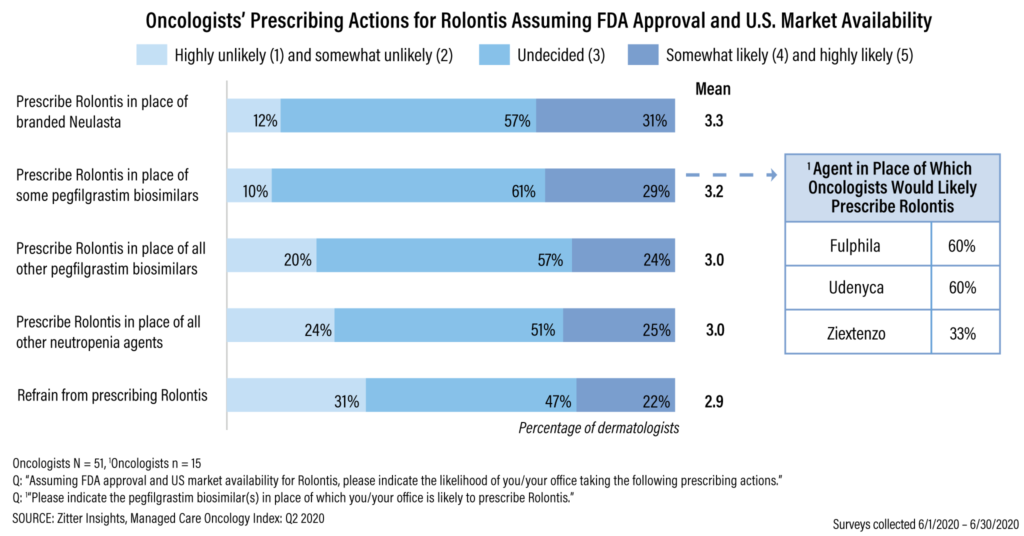
For the Managed Care Oncology Index: Q2 2020, from June 1, 2020, to June 30, 2020, Zitter Insights polled 51 commercial payers covering 129.6 million lives about their management of neutropenia. Once Rolvedon — which previously was known as Rolontis — launches, payers with 51% of lives said they expect to manage the new agent at parity with branded Neulasta.
Zitter Insights and AIS Health are both MMIT companies.
However, respondents with more than three-quarters of lives said it was unlikely that they would prefer the new G-CSF over all other pegfilgrastim agents. Similarly, those covering 83% of lives said it was doubtful that they would prefer Rolvedon over all other neutropenia agents, while those representing 85% of lives said they were not likely to prefer the new product over some pegfilgrastim therapies. Payers covering 20% of lives said that after Rolvedon launches, they likely would not cover Neulasta. Those with 12% of lives said it was possible that they would not cover the new agent.
During the same time frame, Zitter Insights polled 51 oncologists. While many of them were undecided in how they would prescribe Rolvedon and the other neutropenia agents after the new G-CSF launches, 31% said they were likely to prescribe the new agent over Neulasta (see chart). About a quarter of the respondents said it was possible that they would prescribe the new drug in place of all pegfilgrastim biosimilars, as well as in place of all other neutropenia agents.
In a pair of Phase III trials that examined Rolvedon vs. pegfilgrastim, the Spectrum product demonstrated noninferiority to the current market leader in the class in terms of efficacy and safety.
Drugs.com lists the price of one single-dose prefilled syringe of Rolvedon as around $4,748. That’s compared with a wholesale acquisition price of $6,417.99 per dose for Neulasta. A study published in July in the Journal of Managed Care + Specialty Pharmacy found that while patients had lower out-of-pocket costs for two of the biosimilars vs. the reference drug, payers did not have lower expenses for them. Using 2019 IBM MarketScan Commercial and Medicare Supplemental databases, researchers found that health plan costs were $5,783 for Viatris’ Fulphila (pegfilgrastim-jmdb) and $5,845 for Coherus BioSciences, Inc.’s Udenyca (pegfilgrastim-cbqv) compared with $5,618 for Neulasta.
But Neulasta may keep its lead in the space due to an exclusive route of administration: the on-body injector system known as Onpro. The device is filled with Neulasta and attached to the abdomen or back of the arm of a person undergoing chemotherapy. Then, instead of having to return to the provider’s office for administration of Neulasta on the day following chemotherapy, the device automatically administers a dose of the drug. The FDA initially approved the medication as a manual injection in 2002 and then approved the Onpro system for use in 2014. Both formulations are available but are not interchangeable, as there is the potential for giving too much or too little of the recommended dose of Neulasta.
Rolvedon and all six Neulasta biosimilars have been approved for use as a manual subcutaneous injection, although some of the biosimilar manufacturers are developing an on-body injector system. The availability of that system could impact a biosimilar’s uptake “potentially in a positive way, as it now offers a product that can compete with the dosage form that has been unique to Neulasta and in my experience has created challenges for the uptake of Neulasta biosimilars that do not offer that unique dosage form. Many patients and providers have wanted to stick with the brand because of the Onpro dosage form,” says Renee Rayburg RPh., vice president of specialty clinical consulting at Pharmaceutical Strategies Group (PSG), an EPIC company. “Having a biosimilar that will offer this unique dosage form will take away the advantage that today brand Neulasta offers.”
Jeff Stoll, principal and national strategy lead for life sciences at KPMG, agrees: “Patients and physicians have choices. Patients are always going to prefer easy-to-use devices.”
According to PSG’s 2022 Artemetrx State of Specialty Spend and Trend Report, four Neulasta biosimilars had garnered only 39.3% of the pegfilgrastim market share in 2021, up from 31.8% in 2020 and 21.2% in 2019.
However, while Spectrum does not indicate that it has started clinical trials of an on-body system for Rolvedon, it does have a same day as chemotherapy dosing trial underway, the company said in April 2020. Currently all of the long-acting neutropenia agents are administered the day after chemotherapy.
“A same day dosing option would be a unique and meaningful addition to the G-CSF category,” Joe Turgeon, then-president and CEO of Spectrum Pharmaceuticals, said at the time. “We will continue to follow the science and explore ways to add value to patients and health care providers. The initiation
of this study, despite the pandemic, highlights investigator’s interest and our team’s dedication.”
The company’s Form 10-K for 2021 that was filed with the U.S. Securities and Exchange Commission on March 18, 2022, revealed that the clinical trial is in Phase I exploring dosing over 30 minutes. Two other trial arms — one for three-hour dosing and one for five-hour dosing — have been discontinued. “The overall safety profile for the 30-minute arm was similar to what has been seen previously in large randomized studies with G-CSF given 24 hours after chemotherapy,” it stated.
For more information on the Zitter Insights data, contact Jill Brown Kettler at [email protected].
Contact Rayburg via Betsy Van Alstyne at [email protected] and Stoll through Megan B. Miller at [email protected].
