First Humira Biosimilar Hits the Market Today: Should We Be Excited?
Posted on January 23, 2023
Today is a milestone in the world of biosimilars, as the first Humira® biosimilar Amjevita™ (adalimumab-atto), manufactured by Amgen, has launched in the U.S. Will it have the financial impact consumers and payers have hoped for? With so many factors in play it’s hard to say, but let’s take a closer look.
The road to biosimilar acceptance is a journey that began 13 years ago. A brief history:
- 2010: President Obama signed the health care reform Biologics Price Competition and Innovation Act (BPCIA), part of the Affordable Care Act, allowing the FDA to approve biosimilars to be marketed, with the FDA setting the rules as to what is required to gain approval.
- 2012: Biosimilar User Fee Act of 2012 was enacted by Congress, which ensures funding of the FDA to support the timely review of biosimilar applications under the pathway provided by the BPCIA.
- 2015: Zarxio, a biosimilar to Neupogen, became the first biosimilar to be approved in the U.S.
- 2023: As of today, there are 40 FDA-approved biosimilars, 28 of which have been launched.
With the first biosimilar for Humira hitting the market today, here are PSG’s three market predictions for 2023:
- Competition should drive down costs – Humira was first approved for the treatment of rheumatoid arthritis in December 2002, and with the approval of several additional indications, has become the world’s top-selling drug generating more than $20 billion annually in profit for AbbVie. With the increase in utilization and price, Humira has significantly contributed to the growth of the inflammatory drug category which is a top cost driver in pharmacy spend for most plans. Generating competition in a category with significant financial impact for plans is promising. Although Amjevita was approved in 2016 and is the first to launch, there are seven other Humira biosimilars approved and even more in the pipeline. So, competition is expected to be strong over the coming years. We anticipate this will drive lower prices, benefiting both consumers and payers. Although it has taken years, we have seen the biosimilar competition in other drug categories result in just this — lower prices.
- Not all Humira biosimilars are created equal – Humira Biosimilars FDA-Approved.
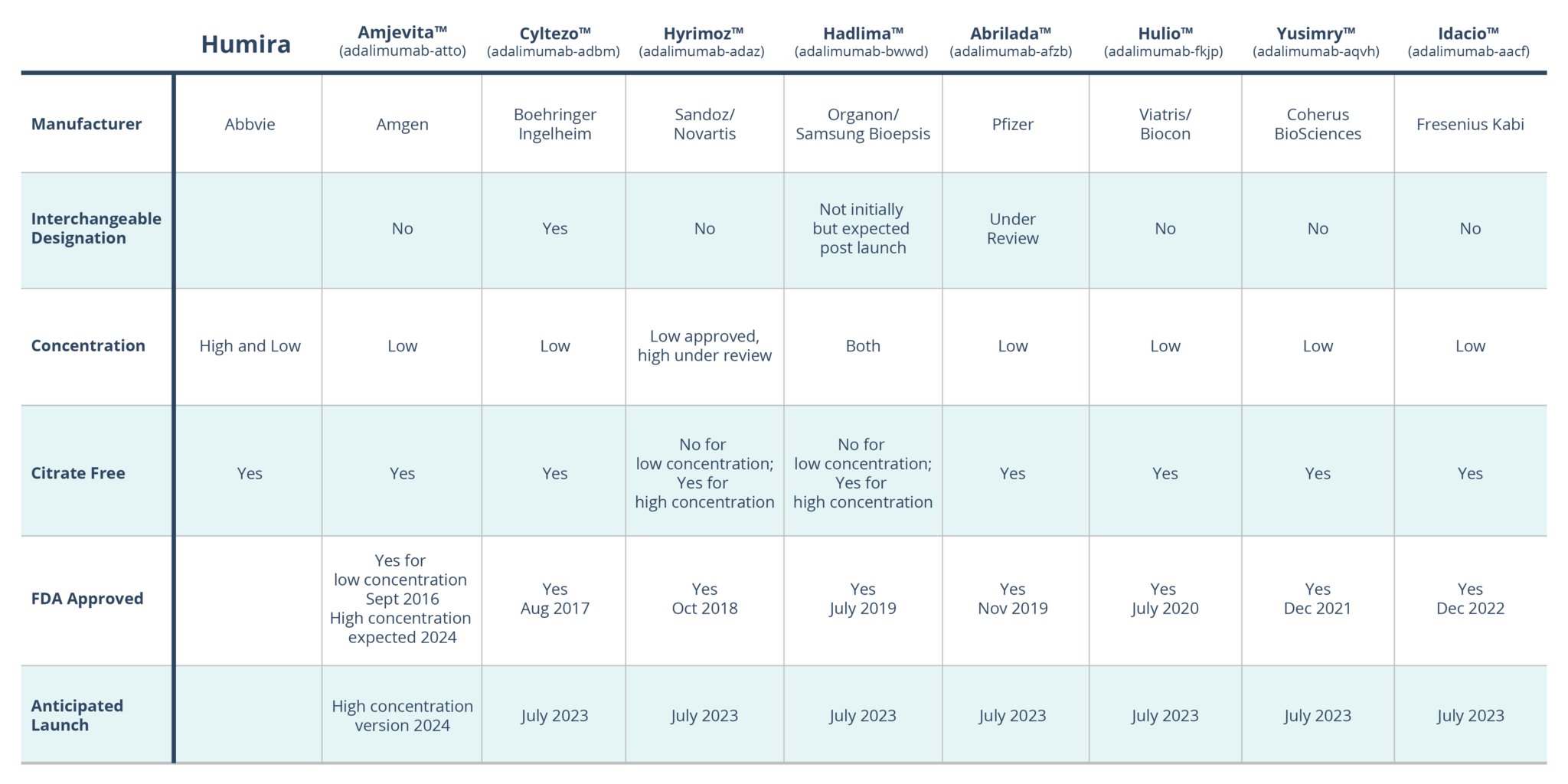
Although there are differences between the Humira biosimilars, each product’s characteristics do not constitute differences in biosimilarity – clinical effectiveness, safety, or quality of the product. However, they may impact a patient’s or provider’s preference. For example, in 2018, AbbVie reformulated Humira and began marketing Humira CF (citrate-free). The new formulation was designed to benefit the patient in three ways: 1) it removed the citrate buffers (responsible for burning upon injection), 2) it was reformulated to a higher concentration, reducing the volume injected by 50%, and 3) it is dosed through a thinner needle. Based on PSG’s current book of business over 80% of Humira utilization is in the citrate-free, high concentration formulation.
Nearly all the currently approved biosimilars will be low concentration formulations, except for Hadlima. Its manufacturer is expected to launch both a low and high formulation in July 2023 or later.
The other difference is the interchangeability status of the biosimilars. An interchangeable designation requires additional research by the manufacturer, and if obtained, allows pharmacists to substitute the biosimilar for Humira without the prescriber’s approval. Biosimilars without this designation will require a prescription for that specific biosimilar product. Cyltezo is the only FDA-approved biosimilar to date with interchangeable status, but only to the original, low concentration formulation of Humira.
Amjevita is approved for the treatment of seven inflammatory conditions including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, psoriatic arthritis, chronic plaque psoriasis, Crohn’s disease, and ulcerative colitis. At launch, it will be available as a citrate-free, low concentration adalimumab delivered via both a smaller and larger gauge needle. So, the only difference between Amjevita and brand Humira will be the concentration. Amjevita will not be interchangeable to Humira, thus it cannot automatically substituted for a prescription written for Humira.
- Driving biosimilar market share
Several PBMs have released their Humira biosimilar formulary strategy, which essentially is to position products with the lowest net cost as preferred. Some PBMs will offer coverage for Humira biosimilars at parity to brand Humira, which means they have committed to continue to cover brand Humira in a preferred position and add some biosimilar(s) in the same preferred position. If Humira continues to be covered in a preferred position and there is no financial incentive for members currently on Humira to switch, the uptake of the Humira biosimilars may be limited. Amgen has announced that they will offer some patient financial support and educational resources for Amjevita. Several other drivers will impact the uptake of Humira biosimilars beyond PBM formulary position:- patients’ awareness of biosimilar options and their costs,
- providers’ awareness and confidence in biosimilar alternatives,
- the impact of different biosimilar formulations and
- adequate biosimilar market supply
Overall, the launch of Amjevita is exciting as the potential genesis of a Humira biosimilar cost-saving revolution. This will become more evident in July when Hadlima, and potentially others, come to market with a high concentration formulation. The interchangeable formula could also build excitement because it allows for more control at the pharmacy level.
At PSG, we will be closely watching the biosimilars trends. Our data-driven analytics system, Artemetrx, provides us with the proprietary ability to monitor the shift in biosimilar utilization as well as the shift in costs. With enhanced capabilities via our biosimilar module, we are committed to following these developments alongside our clients.

