CMS Issues Coverage Guidance on GLP-1 Anti-Obesity Drugs: Implications for Health Plans
Posted on April 5, 2024
CMS Issued Coverage Guidance on GLP-1 Anti-Obesity Drugs Requiring Considerations for Health Plans
GLP-1s (glucagon-like peptide 1 agonist) came up in the news again after the Centers for Medicare and Medicaid Services (CMS) issued coverage guidance on GLP-1 anti-obesity drugs with expanded indications on March 20, 2024.* Since the inception of Medicare Part D, drugs used for weight loss have been excluded from basic coverage. While there has been no change in law, CMS clarified that anti-obesity medications (AOMs) that receive FDA approval for an additional medically accepted indication can be considered a Part D drug for that specific use. This guidance follows the FDA’s approval of Wegovy (semaglutide) for an expanded indication to reduce the risk of major cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke in adults with established cardiovascular disease (CVD) and who are either obese or overweight. These developments will have implications for numerous stakeholders within the industry. It is important to understand how this situation came to be and what considerations to think through going forward.
The Meteoric Rise of GLP-1 Utilization
The impact of GLP-1s on Medicare spending is already remarkable. Based on CMS Medicare Part D Spending by Drug, GLP-1 utilization has increased by approximately 46% annually since 2018 (Compound annual growth rate or CAGR). Medicare Part D claims for Ozempic, Rybelsus, Trulicity, and Mounjaro increased from 1.4 million in 2018 to 8.7 million in 2022 (Figure 1). Spending on Ozempic increased significantly between 2021 and 2022, rising from 10th to 6th place, respectively, in the top-selling Part D drugs.
Figure 1. Medicare Part D Claims for GLP-1s (CY 2018 to 2022)
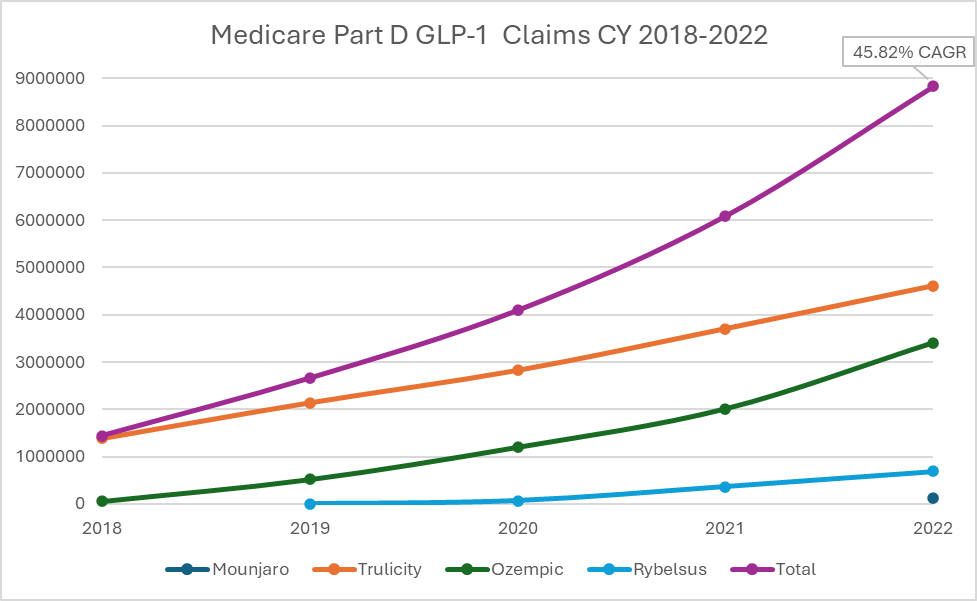
The expanded indication for Wegovy is expected to increase spending on this medication class. The SELECT trial, which supports the expanded indication, enrolled patients 45 years of age and older with pre-existing cardiovascular disease and a body-mass index of 27 or greater without diabetes. Based on these criteria, Novo Nordisk estimates that approximately 10% of people with obesity would meet the indication. PSG estimates that over 5% of the Medicare Part D population is already taking a GLP-1 for diabetes in 2024, and this number could easily double in the coming years. This increase is expected due to potential recommendations from medical organizations like the American College of Cardiology as well as improved member affordability with the implementation of the Inflation Reduction Act and Medicare Prescription Payment Plan in 2025. In addition, the social influence of GLP-1 continues to be normalized.
Traditional and social media influencers, as well as celebrities, have increased the visibility of these drugs for the American public. On March 18th, Oprah kicked off a national conversation on obesity and GLP-1s. Her full endorsement of using Ozempic and Wegovy to treat obesity has opened the door for an important discussion about the use of these drugs in overweight and obese patients. Oprah has found a solution that works for her and is sharing it with the world. Her proven track record of creating awareness and generating interest in products is yet another potential driver of continued demand and use of these products.
GLP-1 Considerations For Health Plans
Financial Implications
Like any other drug being added to CMS’ Formulary Reference File, health plans must consider the holistic impact of adding or not adding Wegovy to formularies. Regardless of approach, members will have a path to coverage for Wegovy on Part D if they use it for the MACE indication.
The first question formulary decision-makers within plans must answer is, “What will be the cost?” PBMs have had challenges negotiating net prices for Wegovy that are equivalent to its sister product, Ozempic, for commercial payers. Based on Novo Nordisk earnings statements, there is approximately a 20% difference in net price between the two products for commercial payers today. We expect the challenge to persist in Medicare. Plans should take into account the likelihood of approval if non-formulary, considering the limited alternative options for this indication and mechanism of action. Manufacturer rebates are not available if the product is not listed on the formulary. It might be best to consider adding Wegovy and managing it appropriately with utilization management (UM). There could also be opportunities for implementing more stringent UM criteria, making the plan ineligible for rebates but providing better trend control overall.
Utilization Management
With this latest action, PBMs will develop coverage criteria for Medicare payers in addition to securing rebates and financial terms with manufacturers. Based on the strength of the clinical data, we expect Wegovy to be added to formularies with utilization management restrictions for prior authorization to prevent off-label use, shorter initial approval periods (e.g., six months approved initially) to ensure efficacy, and quantity limits to reduce stockpiling and waste. Plans that delay formulary addition should expect to start seeing formulary exception request submissions.
Regardless of formulary positioning, ensuring adequate training of health plan staff – and/or oversight of your PBM if delegated – is in place regarding the handling of Wegovy cases will certify appropriate decision-making and appropriately worded member letters. CMS will be critical of plans’ efforts to ensure members have access. Providers will also need education on the appropriate diagnosis to provide when submitting formulary exceptions or prior authorization requests. A significant number of appeals could be overturned if providers only submit a weight loss diagnosis first but then provide the MACE indication on appeal.
Stars Ratings
The addition of Wegovy coverage will be celebrated as a win by members. By adding Wegovy to their Medicare formularies, plans may experience higher patient satisfaction ratings in their CAHPS (Consumer Assessment of Healthcare Providers and Systems) surveys in Rating of Drug Plans (D05 measure) and Getting Needed Prescription Drug (D06 measure).
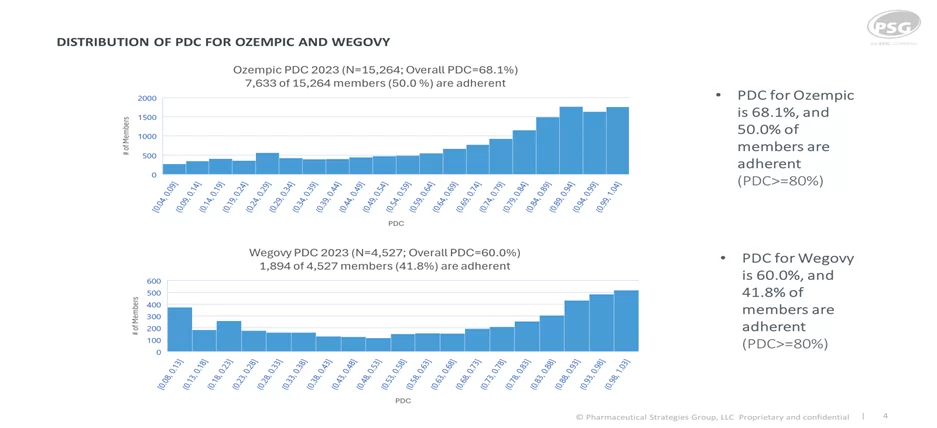
Wegovy is not included in D08 Medicare Adherence for Diabetes Medications since it does not have an FDA-approved indication for diabetes. A positive outcome of adding Wegovy to formularies is the reduction of inappropriate use of Ozempic for indications other than diabetes. We also know that adherence to GLP-1s can be challenging due to their adverse effect profile. Patients using GLP-1s to control diabetes have higher adherence and persistency than GLP-1s used for weight loss. Based on PSG’s Artemetrx analysis for the calendar year 2023, we observed 50% of patients adherent to Ozempic (defined as a proportion of days (PDC) greater than or equal to eighty percent)and an overall PDC of 68%, compared to an adherence rate of 42% and PDC of 60% for Wegovy.
Plan Design
Elevance, Kaiser Permanente, and CVS Health have already announced their intent to add Wegovy to Medicare formularies.
As plans are working with their actuaries and 2025 bids, the financial impacts of the expected increased GLP-1 utilization must be considered in addition to the Part D Redesign plan liability. The impact of Wegovy on the Medicare population may not be as significant as the impact commercial payers have seen when choosing to cover Wegovy, given the concomitant prevalence of diabetes and overweight/obesity in Americans over age 65. Wegovy, like other anti-obesity medications (AOMs), should be used in addition to lifestyle modifications, and ensuring correct population evaluation will be critical to achieving benefits. Plans may wish to offer supplemental benefits such as fitness, healthy meals, and other rewards and incentives that bolster the long-term health effects of AOMs.
Plans should also ensure Wegovy is added to a transition exclusion list to ensure only Part D eligible medically accepted indications are afforded a transition fill. The approved dose of Wegovy is 2.4 mg, so doses of 1.7mg should be flagged for evaluation. Any claims not meeting Medicare coverage rules will need to follow PDE (Prescription Drug Event) deletion procedures.
The increasing use of GLP-1s, along with the recently issued coverage guidance by the CMS, will present several important factors for plans to consider in the future. PSG can assist you in addressing these factors and positioning your plan for success. Connect with an expert to find out more.
*Visit Health Plan Management System (HPMS) on the CMS website to access the emailed PDF (Part_D_Coverage_of_Anti_Obesity_Medications_with_Medically_Accepted_Indications.pdf)

