Five Things Plan Sponsors Need to Know About Specialty Drug Spend and Trend
Posted on September 20, 2021
Would you be surprised to learn specialty drug trend slowed in 2020 compared to recent years?
Unfortunately, we know that the pandemic likely impacted trend because people delayed preventative screenings and starting treatment. For instance, preventative screenings for breast cancer were down 96.6% in April 2020 compared to April 2019.
- Specialty Utilization Rising
- Specialty drug utilization rates continue to rise. Patients using specialty medications are using more of them (8.3% increase in claims perutilizer), even though the percent of all members using specialty medications remained flat.
- Market Traction for Biosimilars
- Biosimilars are driving savings in two ways. First, they cost less than innovator brands. Secondly, they put price pressure on the innovators.
For example, from January 2019 to December 2020, Remicade ASP (Average Sales Price) reduced from $75 per unit to $45 per unit. Unfortunately, the market has not fully recognized the savings opportunities presented by biosimilars. There continue to be interchangeability restrictions that hinder broader adoption.

- Savings Opportunities
- Specialty cost saving opportunities remain in three primary categories:
- Lower cost sites of care
- Review and management of outlier claims
- Utilization management to ensure clinical appropriateness
- Specialty cost saving opportunities remain in three primary categories:
The savings can be significant. For example, shifting just two specialty medications from outpatient hospitals to home infusion or physician offices can save the average plan between $77,811 and $94,403.
- Rare Disease Pipeline
- New therapies for rare diseases such as cystic fibrosis (CF) generate headlines. The special “Investing in Hope” section of PSG’s State of Specialty Spend and Trend Report (page 35) details how Trikafta is changing the lives of those living with CF. Plan sponsors welcome the positive benefits to their populations. Across the PSG book of business, 74% of members experienced decreased medical costs after starting treatment with Trikafta.
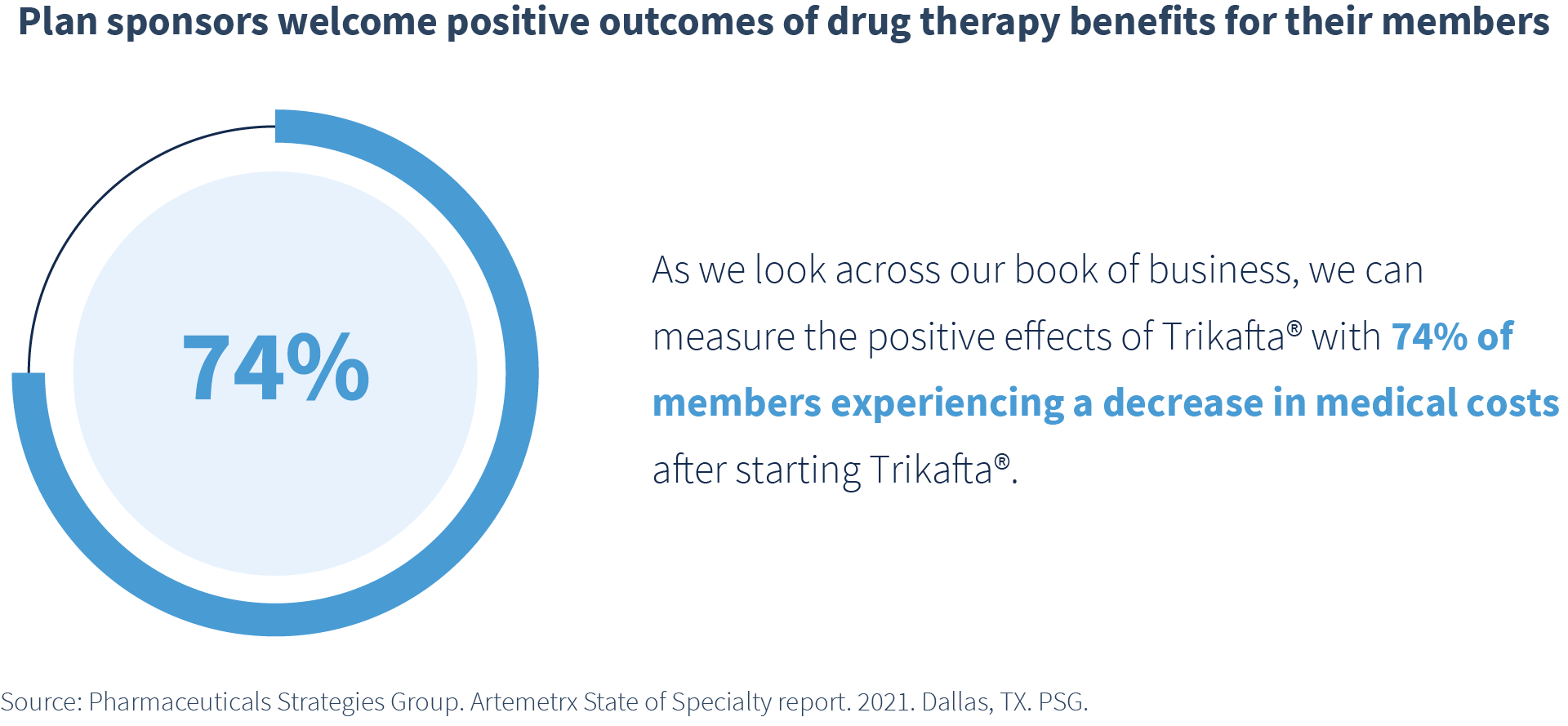
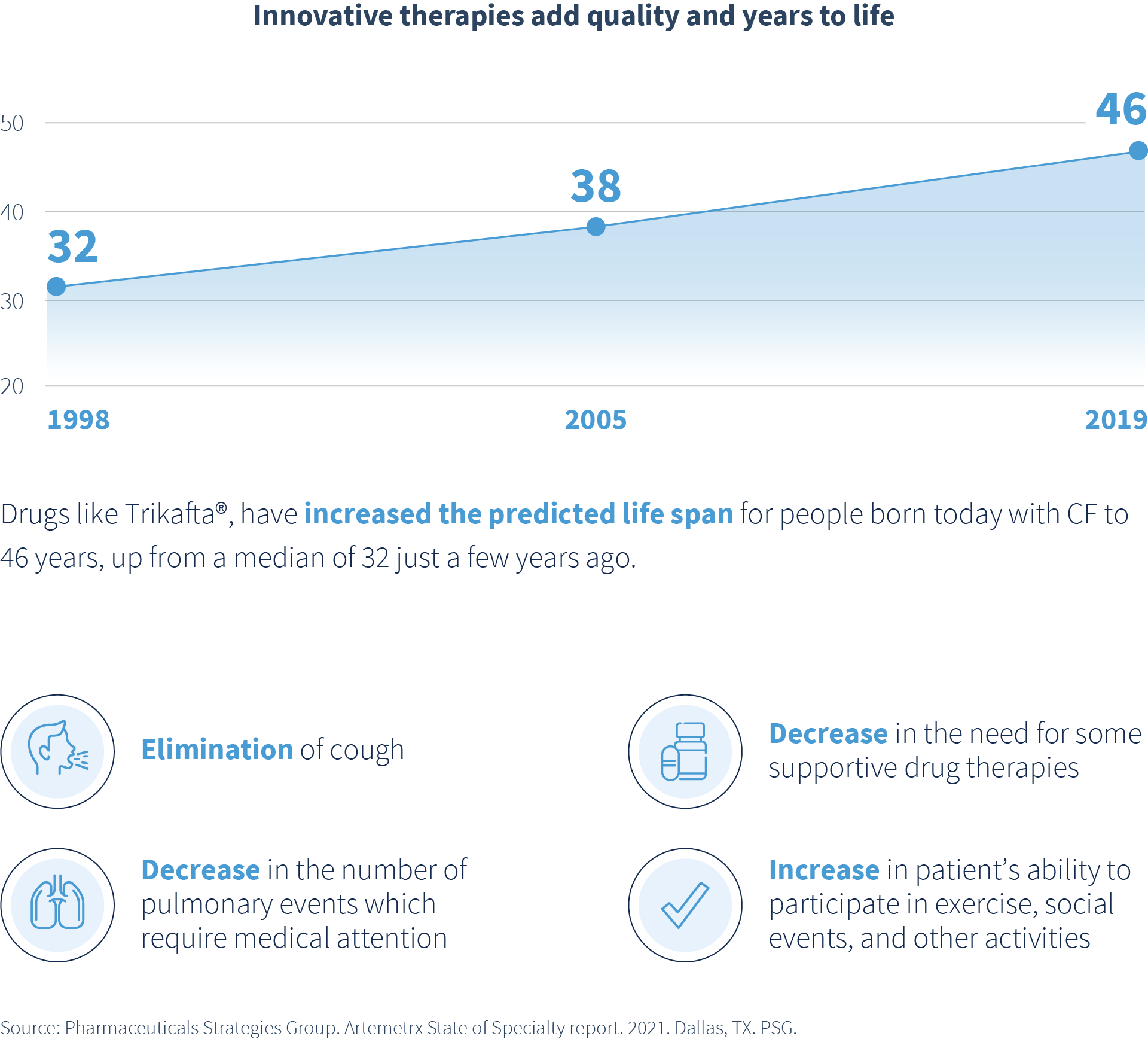
These innovative therapies provide hope for people living with life-threatening conditions. However, only 5% of rare diseases have treatments available, and these treatments come with incredibly high price tags. Yet when available, the improvement of quality of life and added years of life are remarkable.
Download the complete report to learn more


