Betting on Biosimilars
Posted on August 21, 2021
It is a moment more than a decade in the making: biosimilars are finally gaining a notable presence in the biologics market in the U.S.
The U.S. regulatory pathway for biosimilars was paved by the Biologics Price Competition and Innovation Act (BPCIA), initially proposed in 2007 and then passed as part of the Patient Protection and Affordable Care Act of 2010. The first biosimilar in the U.S. to launch back in 2015 was Zarxio® (filgrastim-sndz), as a biosimilar for Neupogen®(filgrastim). In the six years that followed, biosimilar uptake gradually increased as new entrants came to the market.
Today, there are 30 biosimilars FDA approved in the U.S., just a handful not available in the market. The adoption of biosimilars has been positive, with biosimilars gaining market share. For example, biosimilars for Herceptin® (trastuzumab) now make up 40% of all trastuzumab claims, even though the first biosimilar for this biologic did not enter the market until the second half of 2019.
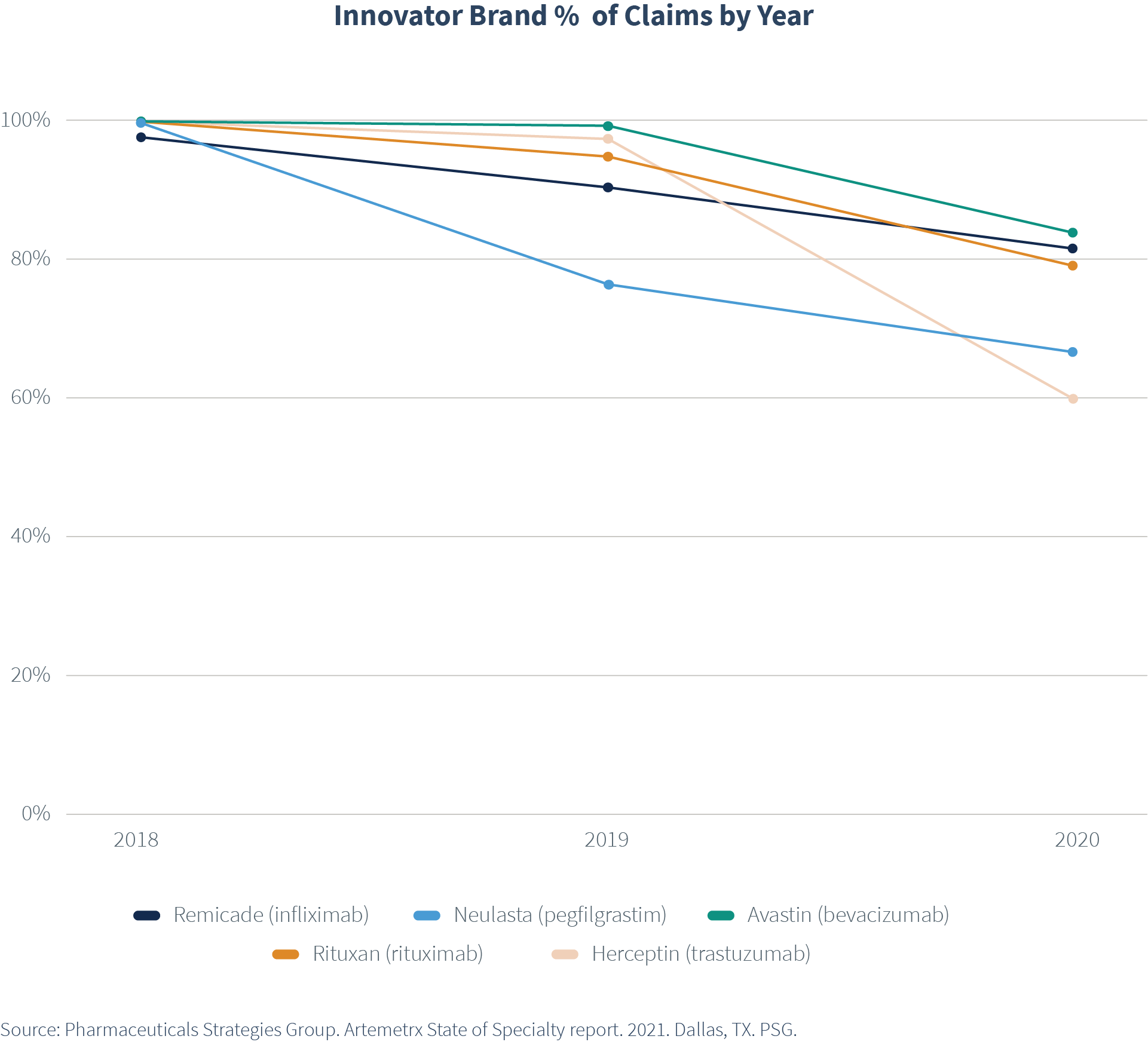
More importantly, however, the result has been what we all hoped for—increased price pressure across both the brands and their biosimilars, resulting in cost savings. The Average Sales Price (ASP) for innovator brands and biosimilars dropped considerably. ASP continues to drop with each new biosimilar entrant.
For example, ASP per unit for Neulasta® (pegfilgrastim) was nearly $4,500 in January 2019, when only one biosimilar was on the market. As the second and third biosimilars entered, ASP dropped for all pegfilgrastim options, with brand Neulasta ASP declining to less than $3,000 by spring of 2021. Further price compression is to be expected as the biosimilars gain additional market share.
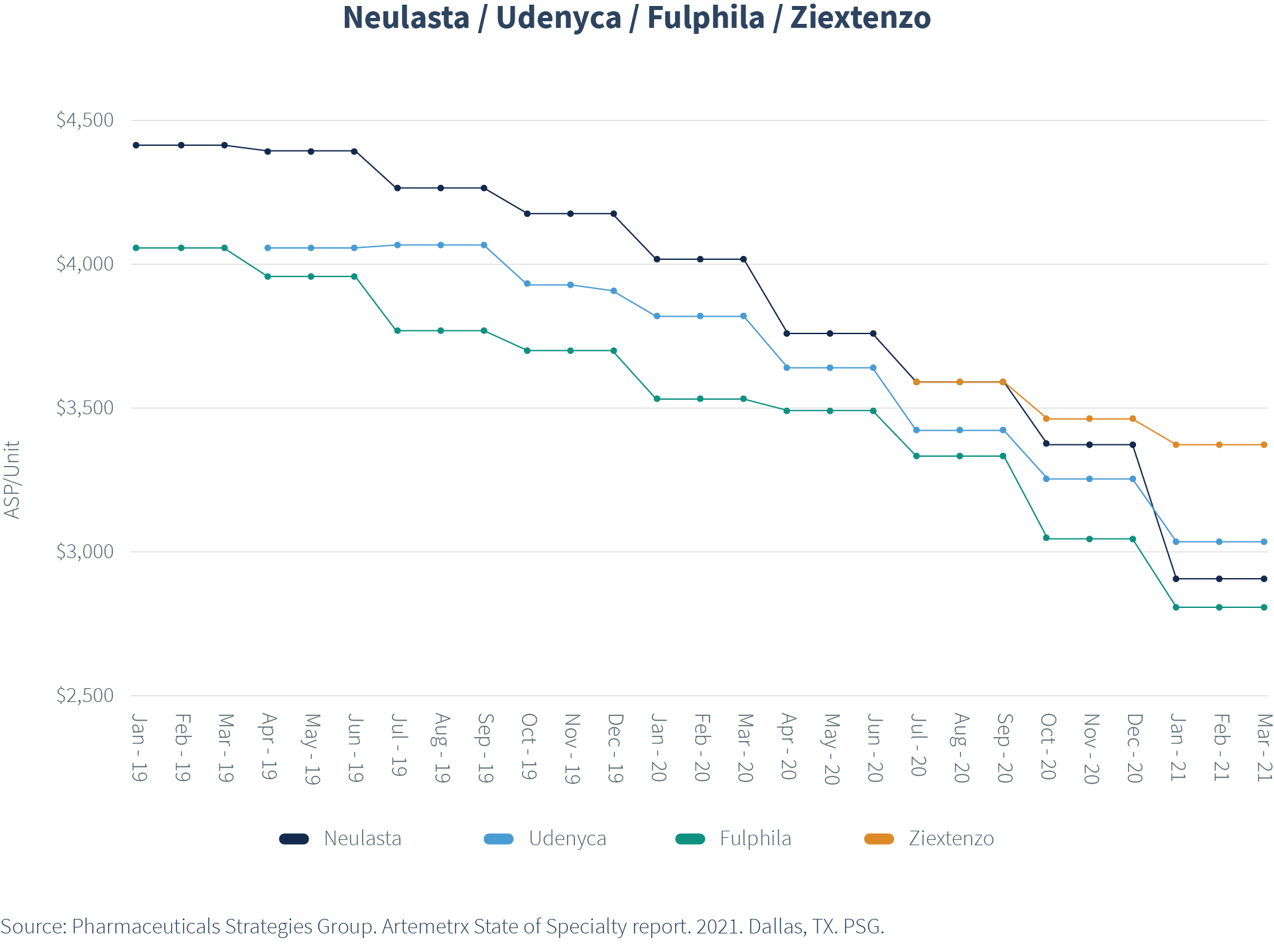
What does this mean in terms of actual dollars for plan sponsors?
Again, using Neulasta as an example, in 2020, plan sponsors saved on average $4,389 annually for each member who moved from the innovator brand to a biosimilar.
What can you do to reap the benefits of biosimilars?
- Use data to guide your strategy
- Identify your utilization and spend for each of the brand innovator drugs for available biosimilars
- Evaluate the pricing, discounts, and rebates offered to identify the lowest net cost product for preferred status
- Pay attention to the biosimilar pipeline and be prepared for new biosimilars gaining FDA-approval
Additional biosimilars are on the horizon for drugs such as Humira® (adalimumab). Humira topped the list of drugs with the highest plan cost in 2020 for another year in the fifth annual State of Specialty Spend and Trend report, averaging $6,900 per claim. Needless to say, the launch of the first biosimilars for Humira in 2023 is a highly anticipated one.
In summary, plan sponsors need to be proactive and partner with their drug benefit consultant, health plan, and PBM to address plan designs to ensure they gain the financial benefits of biosimilars.

