2023 Formulary Changes: Express Scripts
Posted on October 11, 2022
Get the Full Analysis of the Big 3 Formulary Changes:
- Summary of the Big 3 PBM 2023 Formulary Changes
- 2023 Formulary Changes: CVS Health
- 2023 Formulary Changes: OptumRx
Express Scripts released its list of formulary changes effective 1/1/2023 for clients. These changes will apply to National Preferred (NPF) and Flex formularies.
PSG assessed the impact of these changes across our book of business and prepared the following table. Member impact is measured based on the percentage of members from the given PBM currently utilizing one of the newly excluded medications.
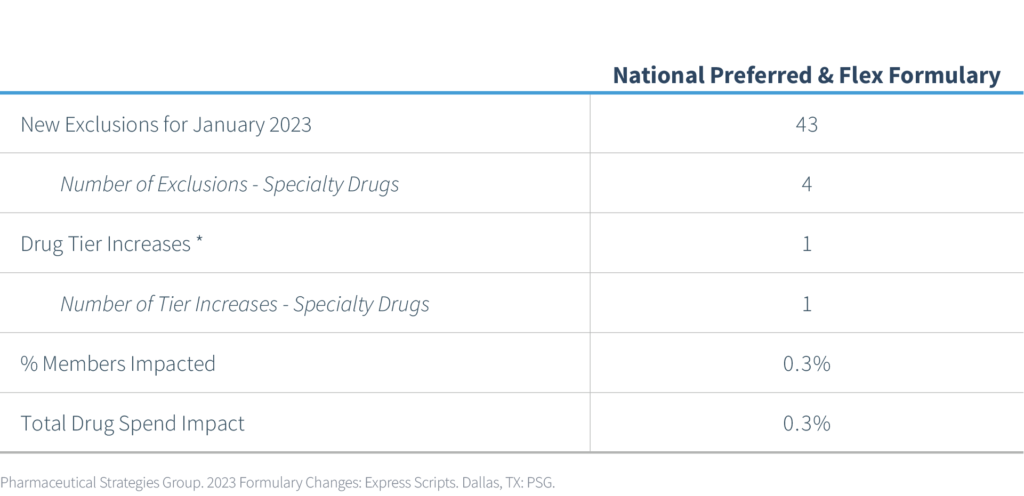
At the beginning of 2022, Express Scripts implemented 518 new exclusions and added 59 more in August 2022. Express Scripts announced 43 new drug exclusions for the National Preferred Formulary, bringing the total number of excluded drugs to 620. According to Express Scripts, the total number of covered drugs is currently at 4,353.
Excluded Drugs
The newly announced 43 excluded drugs are categorized as follows:
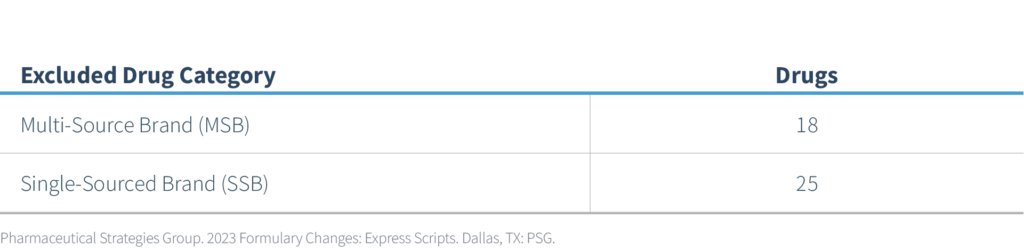
Of the 43 exclusions, 4 are specialty products and 39 are non-specialty. One non-specialty product will move from a preferred status to a non-preferred status. Two categories that may have a more significant impact from a prescription volume and member impact perspective are listed in the following table:

- Incruse Ellipta (umeclidinium inhalation powder) and Spiriva (tiotropium) are currently preferred products and are clinically similar in the maintenance treatment of chronic obstructive pulmonary disease. However, Spiriva does offer some advantages as it is available in two different inhalation dosage forms: 1) Handihaler – capsule for inhalation and 2) Respimat – mist for inhalation. Also, Spiriva is approved for the maintenance treatment of asthma. Spiriva is the market leader, likely lower cost, and will serve as the preferred alternative to Incruse Ellipta.
- Similar to insulin, it is common for PBMs to align formularies to a specific manufacturer’s diabetic supplies. In this case, Express Scripts will prefer Becton Dickinson (BD) syringes and pen needles which are one of the market leaders and likely lower cost. Although comparable to other manufacturers’ diabetic pen needles and syringes, some patients may prefer one brand of diabetic supplies over another. Patient training and education on BD products may be necessary.
Other notable exclusions that may represent incremental cost savings for both plan sponsors and patients are summarized below.
- Fibric Acid Derivatives – this category has long been the target of high-cost/low-value entries. Although fenofibrate has been available as a low-cost generic in multiple strengths for many years, various suppliers have continued to introduce high-cost “branded” generics that provide no incremental clinical value. Lipofen (fenofibrate) is such an example and will, therefore, be excluded in 2023. The more common strengths of the lowest net cost generic fenofibrate products remain as preferred alternatives.
- Idiopathic Pulmonary Fibrosis (IPF) Agents – both the brand tablet and capsule formulations of Esbriet (pirfenidone), a specialty drug used to treat a rare, progressing lung condition called idiopathic pulmonary fibrosis (IPF), will be excluded from the formulary. The tablet formulation is now available generically and will serve as the alternative. Although the utilization of Esbriet for most plans is relatively low, a generic option for a high-cost specialty drug offers the opportunity for cost savings.
- Irritable Bowel Syndrome (IBS) & Chronic Constipation Agents – although Express Scripts named Zelnorm as a new exclusion, its manufacturer Alfasigma voluntarily withdrew Zelnorm from the market in June 2022. Zelnorm was indicated for IBS with constipation. Motegrity (prucalopride), another prokinetic agent, is indicated for chronic idiopathic constipation and is one of the new exclusions announced by Express Scripts. Preferred alternatives in this category include Linzess and Trulance.
- Narcotic Analgesics & Combinations – tramadol has been available as a low-cost generic for several years. However, several higher-cost “branded” generics have been introduced to the market, adding no incremental clinical value. Conzip (tramadol) and other high-cost versions of tramadol are listed as formulary exclusions. The lowest net cost generic versions of tramadol will remain on the formulary as preferred alternatives.
- Multi-Source Brand Specialty Drugs – in addition to Esbriet (pirfenidone), noted above, the multi-source brand specialty drugs listed in the table below are now excluded starting 1/1/2023.
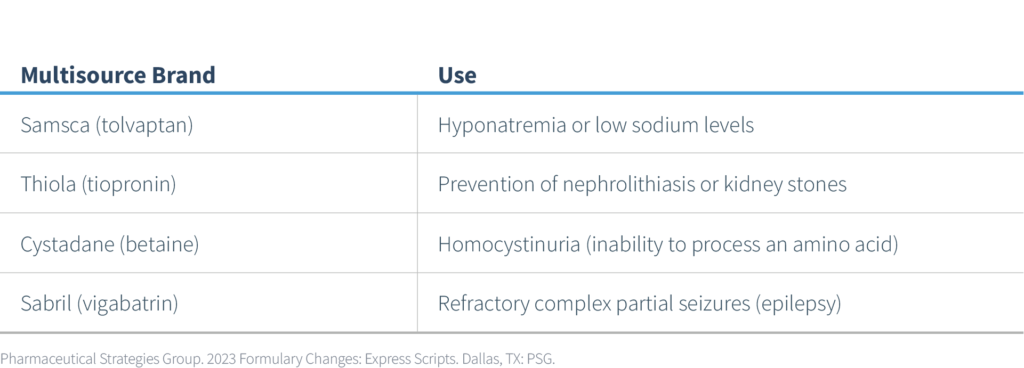
Lower-cost generic equivalents of these branded agents will remain as preferred alternatives. Since these are specialty drugs, any prior authorization requirements for the brand drugs will remain in place for the generic alternatives.

