Insights into the 2022 CVS Formulary Changes
Posted on August 26, 2021
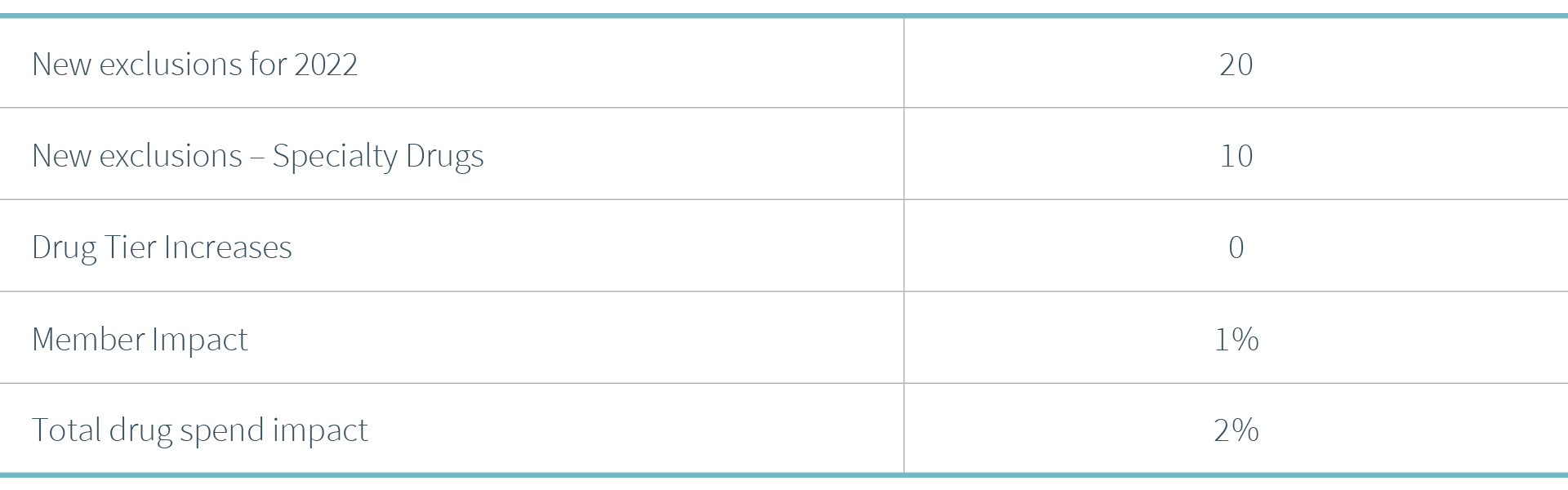
CVS Health (CVS) announced their 2022 formulary changes, both exclusions and additions, stating the focus of their formulary strategy continues to be “providing access to clinically appropriate and cost-effective drugs for plan members at the lowest net cost for our clients.” They expect to deliver $4.4B in client savings while estimating 99.61% of members will not be impacted by the changes.
Key considerations related to these formulary changes include:
• 10 of the 20 new exclusions are for specialty medications
• CVS is expanding use of biosimilars as preferred products
• There is expansion of the CVS brand over generic lower net cost strategy
Across the PSG book of business, the 2022 changes are not expected to have a significant impact, affecting only 1% of members and 2% impact on total drug spend.
Key Clinical Insights
The most considerable formulary change, in terms of member impact, is Eliquis®.
- 72.6% of members impacted are on Eliquis.
- Eliquis is a blood thinner, called Factor Xa inhibitor, an oral tablet taken twice a day and used for the prevention and/or treatment of blood clots. It also reduces the risk of stroke in patients with atrial fibrillation.
- The covered preferred alternatives are Xarelto®, also a Factor Xa inhibitor or warfarin.
- Eliquis entered the market by joining Xarelto as next-generation blood thinners. These are alternatives to warfarin, but unlike warfarin, they do not require any continuous blood monitoring while being used.
- Patients stable on Eliquis will most likely seek to transition to Xarelto, which offers once-daily dosing for most indications.
11.6% of members will be impacted by the exclusion of Aimovig®, the first of a new class of migraine drugs called calcitonin gene-related peptide (CGRP) inhibitors. CGRP inhibitors are the first drugs developed specifically for the prevention of migraines.
- Aimovig is a once-monthly subcutaneous injection used to prevent migraines.
- The preferred alternatives are Ajovy® and Emgality®, which are two other CGRP inhibitors. Ajovy and Emgality are also subcutaneous injections. They are expected to provide the same level of effectiveness.
- In recent weeks, the U.S. Food and Drug Administration (FDA) approved two oral CGRP inhibitors, Qulipta™ and Nurtec ODT™, to prevent episodic migraines.
- It is important to understand the formulary placement of these two new drugs. Their list price may be higher than the injectable CGRP inhibitors, and some members may prefer an oral dosage form over an injectable. This could prove more costly to plan sponsors.
Two biosimilars (Nivestym and Retacrit) will become preferred formulary alternatives with the removal of Leukine® and Aranesp®, respectively.
- While neither of these are biosimilars for these two brand drugs, they are therapeutic alternatives that can be used for the conditions for which Aranesp and Leukine treat.
- Member impact is less than 1%.
In September 2020 the FDA approved generic Truvada® (emtricitabine-tenofovir disoproxil fumurate), which is used for both the treatment and prevention, PrEP (pre-exposure prophylaxis) of HIV.
- As additional generic manufacturers entered the market in the spring of 2021, the list price of the generic has significantly decreased.
- With the exclusion of brand Truvada, transitioning members to the generic should result in cost savings for plan sponsors.
- Similarly, CVS is excluding brand Atripla® (efavirenz-emtricitabine-tenofovir disoproxil fumarate) and Complera® (efavirenz-rilpivirine-disoproxil fumurate), both combination HIV drugs, components of which are available generically.
Could this mark the beginning of attempting to increase the use of lower-cost generic HIV drugs by removing these brand products?
CVS’s brand over generic strategy will continue with the removal of two generics: topical ivermectin 1% cream (Soolantra®) and budesonide ER 9mg tablets (Uceris®), covering their brand counterparts on Tier 1 instead.
- Soolantra is a topical cream used for a dermatologic condition, rosacea.
- Budesonide is an extended-release oral corticosteroid used for the treatment of ulcerative colitis.
- There are lower-cost therapeutic alternatives available for both, including another generic formulation of budesonide extended-release 3mg capsules.
- Utilization and spend for these branded products should be monitored to ensure the lowest-cost products are utilized to treat these conditions.
Cinryze® and Haegarda®, two injectable specialty drugs used to prevent acute attacks of Hereditary Angioedema (HAE), are being excluded leaving two formulary alternatives. One alternative is oral, Orladeyo™, and the other is a subcutaneous injection, Takhzyro®.
- Given HAE is a very rare condition, less than 1% of members across PSG are impacted by this change.
Two disease-modifying therapies for the treatment of multiple sclerosis (MS) are being added back to the formulary, Avonex® and Plegridy® after being excluded effective in 2020.
- These formulary decisions become tricky when members who were stable on these drugs transitioned to previous preferred therapies, and now these previously excluded products are being added back.

